Cialis Full Prescribing Information
(tadalafil) Tablets
Cialis patient information in plain English here
Description
Pharmacology
Indications and Usage
Contraindications
Warnings
Precautions
Drug Interactions
Adverse Reactions
Overdose
Dosage
Supplied
CIALIS® (tadalafil), an oral treatment for erectile dysfunction, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.41. The structural formula is:
The chemical designation is pyrazino[1 ´,2 ´:1,6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-. It is a crystalline solid that is practically insoluble in water and very slightly soluble in ethanol.
CIALIS is available as film-coated, almond-shaped tablets for oral administration. Each tablet contains 5, 10, or 20 mg of tadalafil and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, hypromellose, iron oxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, talc, titanium dioxide, and triacetin.
CLINICAL PHARMACOLOGY
Mechanism of Action
Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle. This response is mediated by the release of nitric oxide (NO) from nerve terminals and endothelial cells, which stimulates the synthesis of cGMP in smooth muscle cells. Cyclic GMP causes smooth muscle relaxation and increased blood flow into the corpus cavernosum. The inhibition of phosphodiesterase type 5 (PDE5) enhances erectile function by increasing the amount of cGMP. Tadalafil inhibits PDE5. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 by tadalafil has no effect in the absence of sexual stimulation.
Studies in vitro have demonstrated that tadalafil is a selective inhibitor of PDE5. PDE5 is found in corpus cavernosum smooth muscle, vascular and visceral smooth muscle, skeletal muscle, platelets, kidney, lung, cerebellum, and pancreas.
In vitro studies have shown that the effect of tadalafil is more potent on PDE5 than on other phosphodiesterases. These studies have shown that tadalafil is >10,000-fold more potent for PDE5 than for PDE1, PDE2, PDE4, and PDE7 enzymes, which are found in the heart, brain, blood vessels, liver, leukocytes, skeletal muscle, and other organs. Tadalafil is >10,000-fold more potent for PDE5 than for PDE3, an enzyme found in the heart and blood vessels. Additionally, tadalafil is 700-fold more potent for PDE5 than for PDE6, which is found in the retina and is responsible for phototransduction. Tadalafil is >9,000-fold more potent for PDE5 than for PDE8, PDE9, and PDE10 and 14-fold more potent for PDE5 than for PDE11A1, an enzyme found in human skeletal muscle. Tadalafil inhibits human recombinant PDE11A1 activity at concentrations within the therapeutic range. The physiological role and clinical consequence of PDE11 inhibition in humans have not been defined.
Pharmacokinetics
Over a dose range of 2.5 to 20 mg, tadalafil exposure (AUC) increases proportionally with dose in healthy subjects. Steady-state plasma concentrations are attained within 5 days of once-daily dosing, and exposure is approximately 1.6-fold greater than after a single dose. Tadalafil is eliminated predominantly by hepatic metabolism, mainly by cytochrome P450 3A4 (CYP3A4). The concomitant use of potent CYP3A4 inhibitors such as ritonavir or ketoconazole resulted in significant increases in tadalafil AUC values (see PRECAUTIONS and DOSAGE AND ADMINISTRATION). Mean tadalafil concentrations measured after the administration of a single oral dose of 20 mg to healthy male subjects are depicted in Figure 1. Figure 1:
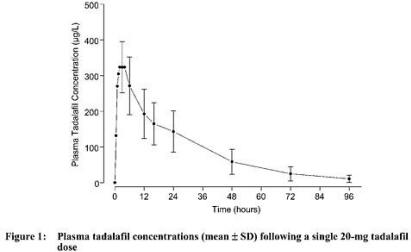
Absorption - After single oral-dose administration, the maximum observed plasma concentration (Cmax) of tadalafil is achieved between 30 minutes and 6 hours (median time of 2 hours). Absolute bioavailability of tadalafil following oral dosing has not been determined.
The rate and extent of absorption of tadalafil are not influenced by food; thus CIALIS may be taken with or without food.
Distribution - The mean apparent volume of distribution following oral administration is approximately 63 L, indicating that tadalafil is distributed into tissues. At therapeutic concentrations, 94% of tadalafil in plasma is bound to proteins.
Less than 0.0005% of the administered dose appeared in the semen of healthy subjects.
Metabolism - Tadalafil is predominantly metabolized by CYP3A4 to a catechol metabolite. The catechol metabolite undergoes extensive methylation and glucuronidation to form the methylcatechol and methylcatechol glucuronide conjugate, respectively. The major circulating metabolite is the methylcatechol glucuronide. Methylcatechol concentrations are less than 10% of glucuronide concentrations. In vitro data suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
Elimination - The mean oral clearance for tadalafil is 2.5 L/hr and the mean terminal half-life is 17.5 hours in healthy subjects. Tadalafil is excreted predominantly as metabolites, mainly in the feces (approximately 61% of the dose) and to a lesser extent in the urine (approximately 36% of the dose).
Pharmacokinetics in Special Populations
Geriatric - Healthy male elderly subjects (65 years or over) had a lower oral clearance of tadalafil, resulting in 25% higher exposure (AUC) with no effect on Cmax relative to that observed in healthy subjects 19 to 45 years of age. No dose adjustment is warranted based on age alone. However, greater sensitivity to medications in some older individuals should be considered (see Geriatric Use under PRECAUTIONS).
Pediatric - Tadalafil has not been evaluated in individuals less than 18 years old.
Hepatic Impairment - In clinical pharmacology studies, tadalafil exposure (AUC) in subjects with mild or moderate hepatic impairment (Child-Pugh Class A or B) was comparable to exposure in healthy subjects when a dose of 10 mg was administered. There are no available data for doses higher than 10 mg of tadalafil in patients with hepatic impairment. Insufficient data are available for subjects with severe hepatic impairment (Child-Pugh Class C). Therefore, for patients with mild or moderate hepatic impairment, the maximum dose should not exceed 10 mg, and use in patients with severe hepatic impairment is not recommended (see DOSAGE AND ADMINISTRATION).
Renal Insufficiency - In clinical pharmacology studies using single-dose tadalafil (5 to 10 mg), tadalafil exposure (AUC) doubled in subjects with mild (creatinine clearance 51 to 80 mL/min) or moderate (creatinine clearance 31 to 50 mL/min) renal insufficiency. In subjects with end-stage renal disease on hemodialysis, there was a two-fold increase in Cmax and 2.7- to 4.1-fold increase in AUC following single-dose administration of 10 or 20 mg tadalafil. Exposure to total methylcatechol (unconjugated plus glucuronide) was 2- to 4-fold higher in subjects with renal impairment, compared to those with normal renal function. Hemodialysis (performed between 24 and 30 hours post-dose) contributed negligibly to tadalafil or metabolite elimination. In a clinical pharmacology study (N=28) at a dose of 10 mg, back pain was reported as a limiting adverse event in male patients with moderate renal impairment. At a dose of 5 mg, the incidence and severity of back pain was not significantly different than in the general population. In patients on hemodialysis taking 10- or 20-mg tadalafil, there were no reported cases of back pain. The dose of tadalafil should be limited to 5 mg not more than once daily in patients with severe renal insufficiency or end-stage renal disease. A starting dose of 5 mg not more than once daily is recommended for patients with moderate renal insufficiency; the maximum recommended dose is 10 mg not more than once in every 48 hours. No dose adjustment is required in patients with mild renal insufficiency (seeDOSAGE AND ADMINISTRATION).
Patients with Diabetes Mellitus - In male patients with diabetes mellitus after a 10 mg tadalafil dose, exposure (AUC) was reduced approximately 19% and Cmax was 5% lower than that observed in healthy subjects. No dose adjustment is warranted.
Pharmacodynamics
Effects on Blood Pressure - Tadalafil 20 mg administered to healthy male subjects produced no significant difference compared to placebo in supine systolic and diastolic blood pressure (difference in the mean maximal decrease of 1.6/0.8 mm Hg, respectively) and in standing systolic and diastolic blood pressure (difference in the mean maximal decrease of 0.2/4.6 mm Hg, respectively). In addition, there was no significant effect on heart rate.
Effects on Blood Pressure when CIALIS is Administered with Nitrates - In clinical pharmacology studies, tadalafil (5 to 20 mg) was shown to potentiate the hypotensive effect of nitrates. Therefore, the use of CIALIS in patients taking any form of nitrates is contraindicated (see CONTRAINDICATIONS).
A study was conducted to assess the degree of interaction between nitroglycerin and tadalafil, should nitroglycerin be required in an emergency situation after tadalafil was taken. This was a double-blind, placebo-controlled, crossover study in 150 male subjects at least 40 years of age (including subjects with diabetes mellitus and/or controlled hypertension) and receiving daily doses of tadalafil 20 mg or matching placebo for 7 days. Subjects were administered a single dose of 0.4 mg sublingual nitroglycerin (NTG) at pre-specified timepoints, following their last dose of tadalafil (2, 4, 8, 24, 48, 72, and 96 hours after tadalafil). The objective of the study was to determine when, after tadalafil dosing, no apparent blood pressure interaction was observed. In this study, a significant interaction between tadalafil and NTG was observed at each timepoint up to and including 24 hours. At 48 hours, by most hemodynamic measures, the interaction between tadalafil and NTG was not observed, although a few more tadalafil subjects compared to placebo experienced greater blood-pressure lowering at this timepoint. After 48 hours, the interaction was not detectable (see Figure 2).
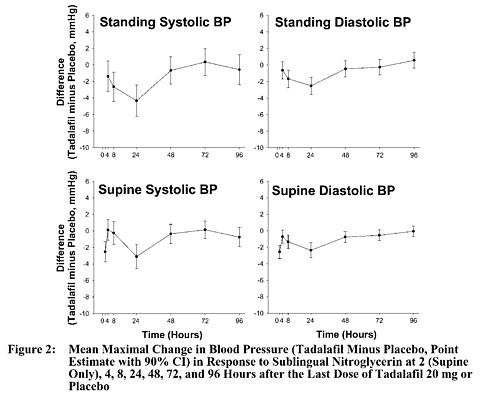
Therefore, CIALIS administration with nitrates is contraindicated. In a patient who has taken CIALIS, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 48 hours should elapse after the last dose of CIALIS before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring (see CONTRAINDICATIONS).
Effects on Exercise Stress Testing - The effects of tadalafil on cardiac function, hemodynamics, and exercise tolerance were investigated in a single clinical pharmacology study. In this blinded crossover trial, 23 subjects with stable coronary artery disease and evidence of exercise-induced cardiac ischemia were enrolled. The primary endpoint was time to cardiac ischemia. The mean difference in total exercise time was 3 seconds (tadalafil 10 mg minus placebo), which represented no clinically meaningful difference. Further statistical analysis demonstrated that tadalafil was non-inferior to placebo with respect to time to ischemia. Of note, in this study, in some subjects who received tadalafil followed by sublingual nitroglycerin in the post-exercise period, clinically significant reductions in blood pressure were observed, consistent with the augmentation by tadalafil of the blood-pressure-lowering effects of nitrates.
Effects on Vision - Single oral doses of phosphodiesterase inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green), using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina. In a study to assess the effects of a single dose of tadalafil 40 mg on vision (N=59), no effects were observed on visual acuity, intraocular pressure, or pupillometry. Across all clinical studies with CIALIS, reports of changes in color vision were rare (<0.1% of patients).
Effects on Sperm Characteristics - There were no clinically relevant effects on sperm concentration, sperm count, motility, or morphology in humans in placebo-controlled studies of daily doses of tadalafil 10 mg (N=204) or 20 mg (N=217) for 6 months. In addition, tadalafil had no effect on serum levels of testosterone, luteinizing hormone, or follicle stimulating hormone.
Effects on Cardiac Electrophysiology - The effect of a single 100-mg dose of tadalafil on the QT interval was evaluated at the time of peak tadalafil concentration in a randomized, double-blinded, placebo, and active (intravenous ibutilide)-controlled crossover study in 90 healthy males aged 18 to 53 years. The mean change in QTc (Fridericia QT correction) for tadalafil, relative to placebo, was 3.5 milliseconds (two-sided 90% CI=1.9, 5.1). The mean change in QTc (Individual QT correction) for tadalafil, relative to placebo, was 2.8 milliseconds (two-sided 90% CI=1.2, 4.4). A 100-mg dose of tadalafil (5 times the highest recommended dose) was chosen because this dose yields exposures covering those observed upon coadministration of tadalafil with potent CYP3A4 inhibitors or those observed in renal impairment. In this study, the mean increase in heart rate associated with a 100-mg dose of tadalafil compared to placebo was 3.1 beats per minute.
Clinical Studies
The efficacy and safety of tadalafil in the treatment of erectile dysfunction has been evaluated in 22 clinical trials of up to 24-weeks duration, involving over 4000 patients. CIALIS, when taken as needed up to once daily, was shown to be effective in improving erectile function in men with erectile dysfunction (ED).
Study Design - CIALIS was studied in the general ED population in 7 randomized, multicenter, double-blinded, placebo-controlled, parallel-arm design, primary efficacy and safety studies of 12-weeks duration. Two of these studies were conducted in the United States and 5 were conducted in centers outside the US. Additional efficacy and safety studies were performed in ED patients with diabetes mellitus and in patients who developed ED status post bilateral nerve-sparing radical prostatectomy.
In these 7 trials, CIALIS was taken as needed, at doses ranging from 2.5 to 20 mg, up to once daily. Patients were free to choose the time interval between dose administration and the time of sexual attempts. Food and alcohol intake were not restricted.
Several assessment tools were used to evaluate the effect of CIALIS on erectile function. The 3 primary outcome measures were the Erectile Function (EF) domain of the International Index of Erectile Function (IIEF) and Questions 2 and 3 from Sexual Encounter Profile (SEP). The IIEF is a 4-week recall questionnaire that was administered at the end of a treatment-free baseline period and subsequently at follow-up visits after randomization. The IIEF EF domain has a 30-point total score, where higher scores reflect better erectile function. SEP is a diary in which patients recorded each sexual attempt made throughout the study. SEP Question 2 asks, "Were you able to insert your penis into your partner's vagina? SEP Question 3 asks, "Did your erection last long enough for you to have successful intercourse? The overall percentage of successful attempts to insert the penis into the vagina (SEP2) and to maintain the erection for successful intercourse (SEP3) is derived for each patient.
Study Results -
ED Population in US Trials - The 2 primary US efficacy and safety trials included a total of 402 men with erectile dysfunction, with a mean age of 59 years (range 27 to 87 years). The population was 78% White, 14% Black, 7% Hispanic, and 1% of other ethnicities, and included patients with ED of various severities, etiologies (organic, psychogenic, mixed), and with multiple co-morbid conditions, including diabetes mellitus, hypertension, and other cardiovascular disease. Most (>90%) patients reported ED of at least 1-year duration. Study A was conducted primarily in academic centers. Study B was conducted primarily in community-based urology practices. In each of these 2 trials, CIALIS 20 mg showed clinically meaningful and statistically significant improvements in all 3 primary efficacy variables (see Table 1). The treatment effect of CIALIS did not diminish over time.
Table 1: Mean Endpoint and change from Baseline for the Primary Efficacy Variables in the Two Primary US Trials
| Study A | Study B | |||||
| Placebo | CIALIS 20 mg | Placebo | CIALIS 20 mg | |||
| (N=49) | (N=146) | p-value | (N=48) | (N=159) | p-value | |
| EF Domain Score | ||||||
| Endpoint | 13.5 | 19.5 | 13.6 | 22.5 | ||
| Change from baseline | -0.2 | 6.9 | <.001 | 0.3 | 9.3 | <.001 |
| Insertion of Penis (SEP2) | ||||||
| Endpoint | 39% | 62% | 43% | 77% | ||
| Change from baseline | 2% | 26% | <.001 | 2% | 32% | <.001 |
| Maintenance of Erection (SEP3) | ||||||
| Endpoint | 25% | 50% | 23% | 64% | ||
| Change from baseline | 5% | 34% | <.001 | 4% | 44% | <.001 |
General ED Population in Trials Outside the US - The 5 primary efficacy and safety studies conducted in the general ED population outside the US included 1112 patients, with a mean age of 59 years (range 21 to 82 years). The population was 76% White, 1% Black, 3% Hispanic, and 20% of other ethnicities, and included patients with ED of various severities, etiologies (organic, psychogenic, mixed), and with multiple co-morbid conditions, including diabetes mellitus, hypertension, and other cardiovascular disease. Most (90%) patients reported ED of at least 1-year duration. In these 5 trials, CIALIS 5, 10, and 20 mg showed clinically meaningful and statistically significant improvements in all 3 primary efficacy variables (see Tables 2, 3, and 4). The treatment effect of CIALIS did not diminish over time.
Table 2: Mean Endpoint and Change from Baseline for the EF Domain of the IIEF in the General ED Population in Five Primary Trials Outside the US
| Placebo | CIALIS 5 mg | CIALIS 10 mg | CIALIS 20 mg | |
| Study C | ||||
| Endpoint [Change from baseline] | 15.0 [0.7] | 17.9 [4.0] | 20.0 [5.6] | |
| p=.006 | p<.001 | |||
| Study D | ||||
| Endpoint [Change from baseline] | 14.4 [1.1] | 17.5 [5.1] | 20.6 [6.0] | |
| p=.002 | p<.001 | |||
| Study E | ||||
| Endpoint [Change from baseline] | 18.1 [2.6] | 22.6 [8.1] | 25.0 [8.0] | |
| p<.001 | p<.001 | |||
| Study F * | ||||
| Endpoint [Change from baseline] | 12.7 [-1.6] | 22.8 [6.8] | ||
| p<.001 | ||||
| Study G | ||||
| Endpoint [Change from baseline] | 14.5 [-0.9] | 21.2 [6.6] | 23.3 [8.0] | |
| p<.001 | p<.001 | |||
| * Treatment duration in Study F was 6 months | ||||
Table 3: Mean Post-Baseline Success Rate and Change from Baseline for SEP Question 2 ("Were you able to insert your penis into the partner's vagina?") in the General ED Population in Five Pivotal Trials Outside the US
| Placebo | CIALIS 5 mg | CIALIS 10 mg | CIALIS 20 mg | |
| Study C | ||||
| Endpoint [Change from baseline] | 49% [6%] | 57% [15%] | 73% [29%] | |
| p=.063 | p<.001 | |||
| Study D | ||||
| Endpoint [Change from baseline] | 46% [2%] | 56% [18%] | 68% [15%] | |
| p=.008 | p<.001 | |||
| Study E | ||||
| Endpoint [Change from baseline] | 55% [10%] | 77% [35%] | 85% [35%] | |
| p<.001 | p<.001 | |||
| Study F * | ||||
| Endpoint [Change from baseline] | 42% [-8%] | 81% [27%] | ||
| p<.001 | ||||
| Study G | ||||
| Endpoint [Change from baseline] | 45% [-6%] | 73% [21%] | 76% [21%] | |
| p<.001 | p<.001 | |||
| * Treatment duration in Study F was 6 months | ||||
Table 4: Mean Post-Baseline Success Rate and Change from Baseline for SEP Question 3 ("Did your erection last long enough for you to have successful intercourse?") in the General ED Population in Five Pivotal Trials Outside the US
| Placebo | CIALIS 5 mg | CIALIS 10 mg | CIALIS 20 mg | |
| Study C | ||||
| Endpoint [Change from baseline] | 26% [4%] | 38% [19%] | 58% [32%] | |
| p=.040 | p<.001 | |||
| Study D | ||||
| Endpoint [Change from baseline] | 28% [4%] | 42% [24%] | 51% [26%] | |
| p<.001 | p<.001 | |||
| Study E | ||||
| Endpoint [Change from baseline] | 43% [15%] | 70% [48%] | 78% [50%] | |
| p<.001 | p<.001 | |||
| Study F * | ||||
| Endpoint [Change from baseline] | 27% [1%] | 74% [40%] | ||
| p<.001 | ||||
| Study G | ||||
| Endpoint [Change from baseline] | 32% [5%] | 57% [33%] | 62% [29%] | |
| p<.001 | p<.001 | |||
| * Treatment duration in Study F was 6 month | ||||
In addition, there were improvements in EF domain scores, success rates based upon SEP Questions 2 and 3, and patient-reported improvement in erections across patients with ED of all degrees of disease severity while taking CIALIS, compared to patients on placebo.
Therefore, in all 7 primary efficacy and safety studies, CIALIS showed statistically significant improvement in patients' ability to achieve an erection sufficient for vaginal penetration and to maintain the erection long enough for successful intercourse, as measured by the IIEF questionnaire and by SEP diaries.
Efficacy in ED Patients with Diabetes Mellitus - CIALIS was shown to be effective in treating ED in patients with diabetes mellitus. Patients with diabetes were included in all 7 primary efficacy studies in the general ED population (N=235) and in 1 study that specifically assessed CIALIS in ED patients with type 1 or type 2 diabetes (N=216). In this randomized, placebo-controlled, double-blinded, parallel-arm design prospective trial, CIALIS demonstrated clinically meaningful and statistically significant improvement in erectile function, as measured by the EF domain of the IIEF questionnaire and Questions 2 and 3 of the SEP diary (see Table 5).
Table 5: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in a Study in ED Patients with Diabetes
| Placebo | CIALIS 10 mg | CIALIS 20 mg | p-value | |
| (N=71) | (N=73) | (N=72) | ||
| EF Domain Score | ||||
| Endpoint [Change from baseline] | 12.2 [0.1] | 19.3 [6.4] | 18.7 [7.3] | <.001 |
| Insertion of Penis (SEP2) | ||||
| Endpoint [Change from baseline] | 30% [-4%] | 57% [22%] | 54% [23%] | <.001 |
| Maintenance of Erection (SEP3) | ||||
| Endpoint [Change from baseline] | 20% [2%] | 48% [28%] | 42% [29%] | <.001 |
Efficacy in ED Patients following Radical Prostatectomy - CIALIS was shown to be effective in treating patients who developed ED following bilateral nerve-sparing radical prostatectomy. In 1 randomized, placebo-controlled, double-blinded, parallel-arm design prospective trial in this population (N=303), CIALIS demonstrated clinically meaningful and statistically significant improvement in erectile function, as measured by the EF domain of the IIEF questionnaire and Questions 2 and 3 of the SEP diary (see Table 6).
Table 6: Mean Endpoint and Change from Baseline for the Primary Efficacy Variables in a Study in Patients who Developed ED Following Bilateral Nerve-Sparing Radical Prostatectomy
| Placebo | CIALIS 20 mg | p-value | |
| (N=102) | (N=201) | ||
| EF Domain Score | |||
| Endpoint [Change from baseline] | 13.3 [1.1] | 17.7 [5.3] | <.001 |
| Insertion of Penis (SEP2) | |||
| Endpoint [Change from baseline] | 32% [2%] | 54% [22%] | <.001 |
| Maintenance of Erection (SEP3) | |||
| Endpoint [Change from baseline] | 19% [4%] | 41% [23%] | <.001 |
Studies to Determine the Optimal Use of CIALIS - Several studies were conducted with the objective of determining the optimal use of CIALIS in the treatment of ED. In one of these studies, the percentage of patients reporting successful erections within 30 minutes of dosing was determined. In this randomized, placebo-controlled, double-blinded trial, 223 patients were randomized to placebo, CIALIS 10, or 20 mg. Using a stopwatch, patients recorded the time following dosing at which a successful erection was obtained. A successful erection was defined as at least 1 erection in 4 attempts that led to successful intercourse. At or prior to 30 minutes, 35% (26/74), 38% (28/74), and 52% (39/75) of patients in the placebo, 10-, and 20-mg groups, respectively, reported successful erections as defined above.
Two studies were conducted to assess the efficacy of CIALIS at a given timepoint after dosing, specifically at 24 hours and at 36 hours after dosing.
In the first of these studies, 348 patients with ED were randomized to placebo or CIALIS 20 mg. Patients were encouraged to make 4 total attempts at intercourse; 2 attempts were to occur at 24 hours after dosing and 2 completely separate attempts were to occur at 36 hours after dosing. The results demonstrated a difference between the placebo group and the CIALIS group at each of the pre-specified timepoints. At the 24-hour timepoint, (more specifically, 22 to 26 hours), 53/144 (37%) patients reported at least 1 successful intercourse in the placebo group versus 84/138 (61%) in the CIALIS 20-mg group. At the 36-hour timepoint (more specifically, 33 to 39 hours), 49/133 (37%) of patients reported at least 1 successful intercourse in the placebo group versus 88/137 (64%) in the CIALIS 20-mg group.
In the second of these studies, a total of 483 patients were evenly randomized to 1 of 6 groups: 3 different dosing groups (placebo, CIALIS 10, or 20 mg) that were instructed to attempt intercourse at 2 different times (24 and 36 hours post-dosing). Patients were encouraged to make 4 separate attempts at their assigned dose and assigned timepoint. In this study, the results demonstrated a statistically significant difference between the placebo group and the CIALIS groups at each of the pre-specified timepoints. At the 24-hour timepoint, the mean, per-patient percentage of attempts resulting in successful intercourse were 42, 56, and 67% for the placebo, CIALIS 10-, and 20-mg groups, respectively. At the 36-hour timepoint, the mean, per-patient percentage of attempts resulting in successful intercourse were 33, 56, and 62% for placebo, CIALIS 10-, and 20-mg groups, respectively.
INDICATIONS AND USAGE
CIALIS is indicated for the treatment of erectile dysfunction.
CONTRAINDICATIONS
Nitrates - Administration of CIALIS to patients who are using any form of organic nitrate, either regularly and/or intermittently, is contraindicated. In clinical pharmacology studies, tadalafil was shown to potentiate the hypotensive effect of nitrates. This is thought to result from the combined effects of nitrates and tadalafil on the nitric oxide/cGMP pathway (see Pharmacodynamics, Effects on Blood Pressure when CIALIS is Administered with Nitrates under CLINICAL PHARMACOLOGY).
Hypersensitivity - CIALIS is contraindicated for patients with a known hypersensitivity to tadalafil or any component of the tablet.
WARNINGS
Cardiovascular
General - Physicians should consider the cardiovascular status of their patients, since there is a degree of cardiac risk associated with sexual activity. Therefore, treatments for erectile dysfunction, including CIALIS, should not be used in men for whom sexual activity is inadvisable as a result of their underlying cardiovascular status.
Left Ventricular Outflow Obstruction - Patients with left ventricular outflow obstruction, (e.g., aortic stenosis and idiopathic hypertrophic subaortic stenosis) can be sensitive to the action of vasodilators, including PDE5 inhibitors.
The following groups of patients with cardiovascular disease were not included in clinical safety and efficacy trials for CIALIS, and, therefore, the use of CIALIS is not recommended in these groups until further information is available:
- patients with a myocardial infarction within the last 90 days
- patients with unstable angina or angina occurring during sexual intercourse
- patients with New York Heart Association Class 2 or greater heart failure in the last 6 months
- patients with uncontrolled arrhythmias, hypotension (170/100 mm Hg)
- patients with a stroke within the last 6 months
In addition, patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials, and use in these patients is not recommended.
Prolonged Erection
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 hours, whether painful or not, should seek emergency medical attention.
PRECAUTIONS
Evaluation of erectile dysfunction should include an appropriate medical assessment to identify potential underlying causes, as well as treatment options.
Before prescribing CIALIS, it is important to note the following:
Alpha-blockers
Caution is advised when PDE5 inhibitors are coadministered with alpha-blockers. PDE5 inhibitors, including CIALIS, and alpha-adrenergic blocking agents are both vasodilators with blood-pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly (see Drug Interactions under PRECAUTIONS), which may lead to symptomatic hypotension (e.g., fainting). Consideration should be given to the following:
- Patients should be stable on alpha-blocker therapy prior to initiating a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors.
- In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest recommended dose.
- In those patients already taking an optimized dose of PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor.
- Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs.
Renal Insufficiency
CIALIS should be limited to 5 mg not more than once daily in patients with severe renal insufficiency or end-stage renal disease. The starting dose of CIALIS in patients with a moderate degree of renal insufficiency should be 5 mg not more than once daily, and the maximum dose should be limited to 10 mg not more than once in every 48 hours. No dose adjustment is required in patients with mild renal insufficiency (see Pharmacokinetics in Special Populations under CLINICAL PHARMACOLOGY).
Hepatic Impairment
In patients with mild or moderate hepatic impairment, the dose of CIALIS should not exceed 10 mg. Because of insufficient information in patients with severe hepatic impairment, use of CIALIS in this group is not recommended (see Pharmacokinetics in Special Populations under CLINICAL PHARMACOLOGY).
Concomitant Use of Potent Inhibitors of Cytochrome P450 3A4 (CYP3A4)
CIALIS is metabolized predominantly by CYP3A4 in the liver. The dose of CIALIS should be limited to 10 mg no more than once every 72 hours in patients taking potent inhibitors of CYP3A4 such as ritonavir, ketoconazole, and itraconazole (see Effects of Other Drugs on CIALIS under Drug Interactions).
General
As with other PDE5 inhibitors, tadalafil has mild systemic vasodilatory properties that may result in transient decreases in blood pressure. In a clinical pharmacology study, tadalafil 20 mg resulted in a mean maximal decrease in supine blood pressure, relative to placebo, of 1.6/0.8 mm Hg in healthy subjects (see Clinical Studies under CLINICAL PHARMACOLOGY). While this effect should not be of consequence in most patients, prior to prescribing CIALIS, physicians should carefully consider whether their patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects. Patients with significant left ventricular outflow obstruction or severely impaired autonomic control of blood pressure may be particularly sensitive to the actions of vasodilators.
The safety and efficacy of combinations of CIALIS and other treatments for erectile dysfunction have not been studied. Therefore, the use of such combinations is not recommended. CIALIS should be used with caution in patients who have conditions that might predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia), or in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie's disease).
When administered in combination with aspirin, tadalafil 20 mg did not prolong bleeding time, relative to aspirin alone. CIALIS has not been administered to patients with bleeding disorders or significant active peptic ulceration. Although CIALIS has not been shown to increase bleeding times in healthy subjects, use in patients with bleeding disorders or significant active peptic ulceration should be based upon a careful risk-benefit assessment and caution.
Physicians should discuss with patients the contraindication of CIALIS with regular and/or intermittent use of organic nitrates. Patients should be counseled that concomitant use of CIALIS with nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke. Physicians should discuss with patients the appropriate action in the event that they experience anginal chest pain requiring nitroglycerin following intake of CIALIS. In such a patient, who has taken CIALIS, where nitrate administration is deemed medically necessary for a life-threatening situation, at least 48 hours should have elapsed after the last dose of CIALIS before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring. Therefore, patients who experience anginal chest pain after taking CIALIS should seek immediate medical attention.
Physicians should advise patients to stop use of all PDE5 inhibitors, including CIALIS, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision, including permanent loss of vision that has been reported rarely postmarketing in temporal association with the use of all PDE5 inhibitors. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or other factors. Physicians should also discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye, including whether such individuals could be adversely affected by use of vasodilators such as PDE5 inhibitors (see Postmarketing surveillance, Ophthalmologic under ADVERSE REACTIONS).
Physicians should discuss with patients the potential for CIALIS to augment the blood-pressure-lowering effect of alpha-blockers and anti-hypertensive medications.
Patients should be made aware that both alcohol and CIALIS, a PDE5 inhibitor, act as mild vasodilators. When mild vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., 5 units or greater) in combination with CIALIS can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache.
Physicians should consider the potential cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Patients who experience symptoms upon initiation of sexual activity should be advised to refrain from further sexual activity and seek immediate medical attention.
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 hours, whether painful or not, should seek emergency medical attention.
The use of CIALIS offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV) should be considered.
Patients should read the patient leaflet entitled "INFORMATION FOR THE PATIENT" before starting therapy with CIALIS and each time the prescription is renewed or refilled.
Drug Interactions
Effects of Other Drugs on CIALIS
Cytochrome P450 Inhibitors
CIALIS is a substrate of and predominantly metabolized by CYP3A4. Studies have shown that drugs that inhibit CYP3A4 can increase tadalafil exposure (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Ketoconazole - Ketoconazole (400 mg daily), a selective and potent inhibitor of CYP3A4, increased tadalafil 20-mg single-dose exposure (AUC) by 312% and Cmax by 22%, relative to the values for tadalafil 20 mg alone. Ketoconazole (200 mg daily) increased tadalafil 10-mg single-dose exposure (AUC) by 107% and Cmax by 15%, relative to the values for tadalafil 10 mg alone.
HIV Protease inhibitor - Ritonavir (200 mg twice daily), an inhibitor of CYP3A4, CYP2C9, CYP2C19, and CYP2D6, increased tadalafil 20-mg single-dose exposure (AUC) by 124% with no change in Cmax, relative to the values for tadalafil 20 mg alone. Although specific interactions have not been studied, other HIV protease inhibitors would likely increase tadalafil exposure (see DOSAGE AND ADMINISTRATION).
Based upon these results, in patients taking concomitant potent CYP3A4 inhibitors, the dose of CIALIS should not exceed 10 mg, and CIALIS should not be taken more frequently than once in every 72 hours (see DOSAGE AND ADMINISTRATION).
Other cytochrome P450 inhibitors - Although specific interactions have not been studied, other CYP3A4 inhibitors, such as erythromycin, itraconazole, and grapefruit juice, would likely increase tadalafil exposure.
Cytochrome P450 Inducers
Studies have shown that drugs that induce CYP3A4 can decrease tadalafil exposure.
Rifampin - Rifampin (600 mg daily), a CYP3A4 inducer, reduced tadalafil 10-mg single-dose exposure (AUC) by 88% and Cmax by 46%, relative to the values for tadalafil 10 mg alone. Although specific interactions have not been studied, other CYP3A4 inducers, such as carbamazepine, phenytoin, and phenobarbitol, would likely decrease tadalafil exposure. No dose adjustment is warranted.
Gastrointestinal Drugs
H2 antagonists - An increase in gastric pH resulting from administration of nizatidine had no significant effect on tadalafil pharmacokinetics.
Antacids - Simultaneous administration of an antacid (magnesium hydroxide/aluminum hydroxide) and tadalafil reduced the apparent rate of absorption of tadalafil without altering exposure (AUC) to tadalafil.
Effects of CIALIS on Other Drugs
Drugs Metabolized by Cytochrome P450
CIALIS is not expected to cause clinically significant inhibition or induction of the clearance of drugs metabolized by cytochrome P450 (CYP) isoforms. Studies have shown that tadalafil does not inhibit or induce P450 isoforms CYP1A2, CYP3A4, CYP2C9, CYP2C19, CYP2D6, and CYP2E1.
CYP1A2 substrate - Tadalafil had no clinically significant effect on the pharmacokinetics of theophylline. When tadalafil was administered to subjects taking theophylline, a small augmentation (3 beats per minute) of the increase in heart rate associated with theophylline was observed.
CYP3A4 substrates - Tadalafil had no clinically significant effect on exposure (AUC) to midazolam or lovastatin.
CYP2C9 substrate - Tadalafil had no clinically significant effect on exposure (AUC) to S-warfarin or R-warfarin, nor did tadalafil affect changes in prothrombin time induced by warfarin.
Alcohol
Alcohol and PDE5 inhibitors, including tadalafil, are mild systemic vasodilators. The interaction of tadalafil with alcohol was evaluated in 3 clinical pharmacology studies. In 2 of these, alcohol was administered at a dose of 0.7 g/kg, which is equivalent to approximately 6 ounces of 80-proof vodka in an 80-kg male, and tadalafil was administered at a dose of 10 mg in 1 study and 20 mg in another. In both these studies, all patients imbibed the entire alcohol dose within 10 minutes of starting. In one of these two studies, blood alcohol levels of 0.08% were confirmed. In these two studies, more patients had clinically significant decreases in blood pressure on the combination of tadalafil and alcohol as compared to alcohol alone. Some subjects reported postural dizziness, and orthostatic hypotension was observed in some subjects. When tadalafil 20 mg was administered with a lower dose of alcohol (0.6 g/kg, which is equivalent to approximately 4 ounces of 80-proof vodka, administered in less than 10 minutes), orthostatic hypotension was not observed, dizziness occurred with similar frequency to alcohol alone, and the hypotensive effects of alcohol were not potentiated.
Tadalafil did not affect alcohol plasma concentrations and alcohol did not affect tadalafil plasma concentrations.
Both alcohol and CIALIS, a PDE5 inhibitor, act as mild vasodilators. When mild vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Substantial consumption of alcohol (e.g., 5 units or greater) in combination with CIALIS can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache.
Anti-Hypertensives
PDE5 inhibitors, including tadalafil, are mild systemic vasodilators. Clinical pharmacology studies were conducted to assess the effect of tadalafil on the potentiation of the blood-pressure-lowering effects of selected anti-hypertensive medications.
Alpha Blockers
Clinical pharmacology studies were conducted to investigate the potential interaction of tadalafil with alpha-blocker agents. In these studies, a single oral dose of tadalafil was administered to healthy male subjects taking daily (at least 7 days duration) oral alpha-blocker. The studies were randomized, double-blinded, crossover designs.
Tamsulosin - A single oral dose of tadalafil 10, 20 mg, or placebo was administered in a 3-period, crossover design to healthy subjects taking 0.4 mg once-daily tamsulosin, a selective alpha[1A]-adrenergic blocker (N=18 subjects). Tadalafil or placebo was administered 2 hours after tamsulosin following a minimum of seven days of tamsulosin dosing.
Table 7: Tamsulosin Study: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
| Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg) | Tadalafil 10 mg | Tadalafil 20 mg |
| Supine | 3.2 (-2.3, 8.6) | 3.2 (-2.3, 8.7) |
| Standing | 1.7 (-4.7, 8.1) | 2.3 (-4.1, 8.7) |
Blood pressure was measured manually at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours after tadalafil or placebo dosing. There were 2, 2, and 1 outliers (subjects with a decrease from baseline in standing systolic blood pressure of >30 mm Hg at one or more time points) following administration of tadalafil 10 mg, 20 mg, and placebo, respectively. There were no subjects with a standing systolic blood pressure <85 mm Hg. No severe adverse events potentially related to blood-pressure effects were reported. No syncope was reported.
Doxazosin - Two clinical pharmacology studies were conducted with tadalafil and doxazosin, an alpha[1]-adrenergic blocker.
In the first doxazosin study, a single oral dose of tadalafil 20 mg or placebo was administered in a 2-period, crossover design to healthy subjects taking oral doxazosin 8 mg daily (N=18 subjects). Doxazosin was administered at the same time as tadalafil or placebo after a minimum of seven days of doxazosin dosing.
Table 8: Doxazosin Study 1: Mean Maximal Decrease (95% CI) in Systolic Blood Pressure
| Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg) | Tadalafil 20 mg |
| Supine | 3.6 (-1.5, 8.8) |
| Standing | 9.8 (4.1, 15.5) |
Blood pressure was measured manually at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours after tadalafil or placebo administration. Outliers were defined as subjects with a standing systolic blood pressure of 30 mm Hg at one or more time points. There were 9 and 3 outliers following administration of tadalafil 20 mg and placebo, respectively. Five and two subjects were outliers due to a decrease from baseline in standing systolic BP of >30 mm Hg, while five and one subject were outliers due to standing systolic BP <85 mm Hg following tadalafil and placebo, respectively. Severe adverse events potentially related to blood-pressure effects were assessed. No such events were reported following placebo. Two such events were reported following administration of tadalafil. Vertigo was reported in one subject that began 7 hours after dosing and lasted about 5 days. This subject previously experienced a mild episode of vertigo on doxazosin and placebo. Dizziness was reported in another subject that began 25 minutes after dosing and lasted 1 day. No syncope was reported.
In the second doxazosin study, a single oral dose of tadalafil 20 mg was administered to healthy subjects taking oral doxazosin, either 4 or 8 mg daily. The study (N=72 subjects) was conducted in three parts, each a 3-period crossover.
In part A (N=24), subjects were titrated to doxazosin 4 mg administered daily at 8 a.m. Tadalafil was administered at either 8 a.m., 4 p.m., or 8 p.m. There was no placebo control.
In part B (N=24), subjects were titrated to doxazosin 4 mg administered daily at 8 p.m. Tadalafil was administered at either 8 a.m., 4 p.m., or 8 p.m. There was no placebo control.
In part C (N=24), subjects were titrated to doxazosin 8 mg administered daily at 8 a.m. In this part, tadalafil or placebo were administered at either 8 a.m. or 8 p.m.
The placebo-subtracted mean maximal decreases in systolic blood pressure over a 12-hour period after dosing in the placebo-controlled portion of the study (part C) are shown in the following table.
Table 9: Doxazosin Study 2 (Part C): Mean Maximal Decrease in Systolic Blood Pressure
| Placebo-subtracted mean maximal decrease in systolic blood pressure (mm Hg) | Tadalafil 20 mg at 8 a.m. | Tadalafil 20 mg at 8 p.m. |
| Ambulatory Blood-Pressure Monitoring (ABPM) | 7 | 8 |
Blood Pressure Blood pressure was measured by ABPM every 15 to 30 minutes for up to 36 hours after tadalafil or placebo. Subjects were categorized as outliers if one or more systolic blood pressure readings of 30 mm Hg from a time-matched baseline occurred during the analysis interval.
Of the 24 subjects in part C, 16 subjects were categorized as outliers following administration of tadalafil and 6 subjects were categorized as outliers following placebo during the 24-hour period after 8 a.m. dosing of tadalafil or placebo. Of these, 5 and 2 were outliers due to systolic BP 30 mm Hg following tadalafil and placebo, respectively.
During the 24-hour period after 8 p.m. dosing, 17 subjects were categorized as outliers following administration of tadalafil and 7 subjects following placebo. Of these, 10 and 2 subjects were outliers due to systolic BP 30 mm Hg, following tadalafil and placebo, respectively.
Some additional subjects in both the tadalafil and placebo groups were categorized as outliers in the period beyond 24 hours.
Severe adverse events potentially related to blood-pressure effects were assessed. In the study (N=72 subjects), 2 such events were reported following administration of tadalafil (symptomatic hypotension in one subject that began 10 hours after dosing and lasted approximately 1 hour, and dizziness in another subject that began 11 hours after dosing and lasted 2 minutes). No such events were reported following placebo. In the period prior to tadalafil dosing, one severe event (dizziness) was reported in a subject during the doxazosin run-in phase.
Other Anti-Hypertensive Agents
Amlodipine - A study was conducted to assess the interaction of amlodipine (5 mg daily) and tadalafil 10 mg. There was no effect of tadalafil on amlodipine blood levels and no effect of amlodipine on tadalafil blood levels. The mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking amlodipine was 3/2 mm Hg, compared to placebo. In a similar study using tadalafil 20 mg, there were no clinically significant differences between tadalafil and placebo in subjects taking amlodipine.
Metoprolol - A study was conducted to assess the interaction of sustained-release metoprolol (25 to 200 mg daily) and tadalafil 10 mg. Following dosing, the mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking metoprolol was 5/3 mm Hg, compared to placebo.
Bendrofluazide - A study was conducted to assess the interaction of bendrofluazide (2.5 mg daily) and tadalafil 10 mg. Following dosing, the mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking bendrofluazide was 6/4 mm Hg, compared to placebo.
Enalapril - A study was conducted to assess the interaction of enalapril (10 to 20 mg daily) and tadalafil 10 mg. Following dosing, the mean reduction in supine systolic/diastolic blood pressure due to tadalafil 10 mg in subjects taking enalapril was 4/1 mm Hg, compared to placebo.
Angiotensin II receptor blocker (and other anti-hypertensives) - A study was conducted to assess the interaction of angiotensin II receptor blockers and tadalafil 20 mg. Subjects in the study were taking any marketed angiotensin II receptor blocker, either alone, as a component of a combination product, or as part of a multiple anti-hypertensive regimen. Following dosing, ambulatory measurements of blood pressure revealed differences between tadalafil and placebo of 8/4 mm Hg in systolic/diastolic blood pressure.
Aspirin
Tadalafil did not potentiate the increase in bleeding time caused by aspirin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Tadalafil was not carcinogenic to rats or mice when administered daily for 2 years at doses up to 400 mg/kg/day. Systemic drug exposures, as measured by AUC of unbound tadalafil, were approximately 10-fold for mice, and 14- and 26-fold for male and female rats, respectively, the exposures in human males given Maximum Recommended Human Dose (MRHD) of 20 mg.
Tadalafil was not mutagenic in the in vitro bacterial Ames assays or the forward mutation test in mouse lymphoma cells. Tadalafil was not clastogenic in the in vitro chromosomal aberration test in human lymphocytes or the in vivo rat micronucleus assays.
There were no effects on fertility, reproductive performance or reproductive organ morphology in male or female rats given oral doses of tadalafil up to 400 mg/kg/day, a dose producing AUCs for unbound tadalafil of 14-fold for males or 26-fold for females the exposures observed in human males given the MRHD of 20 mg. In beagle dogs given tadalafil daily for 3 to 12 months, there was treatment-related non-reversible degeneration and atrophy of the seminiferous tubular epithelium in the testes in 20-100% of the dogs that resulted in a decrease in spermatogenesis in 40-75% of the dogs at doses of ≥10 mg/kg/day. Systemic exposure (based on AUC) at no-observed-adverse-effect-level (NOAEL) (10 mg/kg/day) for unbound tadalafil was similar to that expected in humans at the MRHD of 20 mg.
There were no treatment-related testicular findings in rats or mice treated with doses up to 400 mg/kg/day for 2 years.
In men, there were no clinically relevant effects on sperm concentration, sperm count, motility, or morphology in placebo-controlled studies of daily doses of tadalafil 10 mg (N=204) or 20 mg (N=217) for 6 months. In addition, tadalafil had no effect on serum levels of testosterone, luteinizing hormone, or follicle stimulating hormone in males.
Animal Toxicology
Animal studies showed vascular inflammation in tadalafil-treated mice, rats, and dogs. In mice and rats, lymphoid necrosis and hemorrhage were seen in the spleen, thymus, and mesenteric lymph nodes at unbound tadalafil exposure of 2- to 33-fold above the human exposure (AUCs) at the MRHD of 20 mg. In dogs, an increased incidence of disseminated arteritis was observed in 1- and 6-month studies at unbound tadalafil exposure of 1- to 54-fold above the human exposure (AUC) at the MRHD of 20 mg. In a 12-month dog study, no disseminated arteritis was observed, but 2 dogs exhibited marked decreases in white blood cells (neutrophils) and moderate decreases in platelets with inflammatory signs at unbound tadalafil exposures of approximately 14- to 18-fold the human exposure at the MRHD of 20 mg. The abnormal blood-cell findings were reversible within 2 weeks upon removal of the drug.
Pregnancy, Nursing Mothers, and Pediatric
Use CIALIS is not indicated for use in newborns, children, or women.
Tadalafil and/or its metabolites cross the placenta, resulting in fetal exposure in rats. Tadalafil and/or its metabolites were secreted into the milk in lactating rats at concentrations approximately 2.4-fold greater than found in the plasma. Following a single-oral dose of 10 mg/kg, approximately 0.1% of the total radioactive dose was excreted into the milk within 3 hours. It is not known if tadalafil and/or its metabolites is excreted in human breast milk. Use of tadalafil in nursing mothers is not recommended.
Pregnancy Category B - There was no evidence of teratogenicity, embryotoxicity, or fetotoxicity in rat or mouse fetuses that received up to 1000 mg/kg/day during the major organ development. Plasma exposure at this dose is approximately 11-fold greater than the AUC values for unbound tadalafil in humans given the MRHD of 20 mg. In a rat prenatal and postnatal development study at doses of 60, 200, and 1000 mg/kg, there was a reduction in postnatal survival of pups. The no-observed-effect-level (NOEL) for maternal toxicity was 200 mg/kg/day and for developmental toxicity was 30 mg/kg/day, which gives approximately 16- and 10-fold exposure multiples, respectively, of the human AUC for the MRHD dose of 20 mg. There are no adequate and well-controlled studies of tadalafil in pregnant women.
Geriatric Use
Approximately 25% of patients in the primary efficacy and safety studies of tadalafil were greater than 65 years of age. No overall differences in efficacy and safety were observed between older and younger patients. No dose adjustment is warranted based on age alone. However, greater sensitivity to medications in some older individuals should be considered (see Special Populations under CLINICAL PHARMACOLOGY).
ADVERSE REACTIONS
Tadalafil was administered to over 5700 men (mean age 59, range 19 to 87 years) during clinical trials worldwide. Over 1000 patients were treated for 1 year or longer and over 1300 patients were treated for 6 months or more.
In placebo-controlled Phase 3 clinical trials, the discontinuation rate due to adverse events in patients treated with tadalafil 10 or 20 mg was 3.1%, compared to 1.4% in placebo-treated patients.
When tadalafil was taken as recommended in the placebo-controlled clinical trials, the following adverse events were reported (see Table 10):
Table 7: Treatment-Emergent Adverse Events Reported by >/=2% of Patients Treated with Tadalafil (10 or 20 mg) and More Frequent on Drug than Placebo in the Eight Primary Placebo- Controlled Phase 3 Studies (Including a Study in Patients with Diabetes)
| Adverse Event | Placebo | Tadalafil 5 mg | Tadalafil 10 mg | Tadalafil 20 mg |
| (N=476) | (N=151) | (N=394) | (N=635) | |
| Headache | 5% | 11% | 11% | 15% |
| Dyspepsia | 1% | 4% | 8% | 10% |
| Back pain | 3% | 3% | 5% | 6% |
| Myalgia | 1% | 1% | 4% | 3% |
| Nasal congestion | 1% | 2% | 3% | 3% |
| Flushing * | 1% | 2% | 3% | 3% |
| Pain in limb | 1% | 1% | 3% | 3% |
| * The term flushing includes: facial flushing and flushing | ||||
Back pain or myalgia was reported at incidence rates described in Table 10. In tadalafil clinical pharmacology trials, back pain or myalgia generally occurred 12 to 24 hours after dosing and typically resolved within 48 hours. The back pain/myalgia associated with tadalafil treatment was characterized by diffuse bilateral lower lumbar, gluteal, thigh, or thoracolumbar muscular discomfort and was exacerbated by recumbancy. In general, pain was reported as mild or moderate in severity and resolved without medical treatment, but severe back pain was reported infrequently (<5% of all reports). When medical treatment was necessary, acetaminophen or non-steroidal anti-inflammatory drugs were generally effective; however, in a small percentage of subjects who required treatment, a mild narcotic (e.g., codeine) was used. Overall, approximately 0.5% of all tadalafil-treated subjects discontinued treatment as a consequence of back pain/myalgia. Diagnostic testing, including measures for inflammation, muscle injury, or renal damage revealed no evidence of medically significant underlying pathology.
Across all studies with any tadalafil dose, reports of changes in color vision were rare (<0.1% of patients).
The following section identifies additional, less frequent events (<2%) reported in controlled clinical trials; a causal relationship of these events to CIALIS is uncertain. Excluded from this list are those events that were minor, those with no plausible relation to drug use, and reports too imprecise to be meaningful:
Body as a whole: asthenia, face edema, fatigue, pain
Cardiovascular: angina pectoris, chest pain, hypotension, hypertension, myocardial infarction, postural hypotension, palpitations, syncope, tachycardia
Digestive: abnormal liver function tests, diarrhea, dry mouth, dysphagia, esophagitis, gastroesophageal reflux, gastritis, GGTP increased, loose stools, nausea, upper abdominal pain, vomiting
Musculoskeletal: arthralgia, neck pain
Nervous: dizziness, hypesthesia, insomnia, paresthesia, somnolence, vertigo
Respiratory: dyspnea, epistaxis, pharyngitis
Skin and Appendages: pruritus, rash, sweating
Ophthalmologic: blurred vision, changes in color vision, conjunctivitis (including conjunctival hyperemia), eye pain, lacrimation increase, swelling of eyelids
Urogenital: erection increased, spontaneous penile erection
Postmarketing surveillance
Cardiovascular and cerebrovascular: Serious cardiovascular events, including myocardial infarction, sudden cardiac death, stroke, chest pain, palpitations, and tachycardia, have been reported postmarketing in temporal association with the use of tadalafil. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of CIALIS without sexual activity. Others were reported to have occurred hours to days after the use of CIALIS and sexual activity. It is not possible to determine whether these events are related directly to CIALIS, to sexual activity, to the patient's underlying cardiovascular disease, to a combination of these factors, or to other factors (see WARNINGS for additional information).
Other adverse events: The following list includes other adverse events that have been identified during postmarketing use of CIALIS. The list does not include adverse events that are reported from clinical trials and that are listed elsewhere in this section. These events have been chosen for inclusion either due to their seriousness, reporting frequency, lack of clear alternative causation, or a combination of these factors. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a whole: hypersensitivity reactions including urticaria, Stevens-Johnson syndrome, and exfoliative dermatitis
Ophthalmologic: visual field defect, retinal vein occlusion
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely postmarketing in temporal association with the use of phosphodiesterase type 5 (PDE5) inhibitors, including CIALIS. Most, but not all, of these patients had underlying anatomic or vascular risk factors for development of NAION, including but not necessarily limited to: low cup to disc ratio ("crowded disc"), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia, and smoking. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors, to the patient's underlying vascular risk factors or anatomical defects, to a combination of these factors, or to other factors (see Information for Patients under PRECAUTIONS).
Urogenital: priapism (see WARNINGS)
OVERDOSAGE
Single doses up to 500 mg have been given to healthy subjects, and multiple daily doses up to 100 mg have been given to patients. Adverse events were similar to those seen at lower doses. In cases of overdose, standard supportive measures should be adopted as required. Hemodialysis contributes negligibly to tadalafil elimination.
DOSAGE AND ADMINISTRATION
The recommended starting dose of CIALIS in most patients is 10 mg, taken prior to anticipated sexual activity. The dose may be increased to 20 mg or decreased to 5 mg, based on individual efficacy and tolerability. The maximum recommended dosing frequency is once per day in most patients.
CIALIS was shown to improve erectile function compared to placebo up to 36 hours following dosing. Therefore, when advising patients on optimal use of CIALIS, this should be taken into consideration.
CIALIS may be taken without regard to food.
Renal Insufficiency - No dose adjustment is required in patients with mild renal insufficiency. For patients with moderate (creatinine clearance 31 to 50 mL/min) renal insufficiency, a starting dose of 5 mg not more than once daily is recommended, and the maximum dose should be limited to 10 mg not more than once in every 48 hours. For patients 23 with severe (creatinine clearance <30 mL/min) renal insufficiency on hemodialysis, the maximum recommended dose is 5 mg (see General and Patients with Renal Insufficiency under PRECAUTIONS and Pharmacokinetics in Special Populations under CLINICAL PHARMACOLOGY).
Hepatic Impairment - For patients with mild or moderate degrees of hepatic impairment (Child-Pugh Class A or B), the dose of CIALIS should not exceed 10 mg once daily. In patients with severe hepatic impairment (Child-Pugh Class C), the use of CIALIS is not recommended (see Patients with Hepatic Impairment under PRECAUTIONS and Pharmacokinetics in Special Populations under CLINICAL PHARMACOLOGY).
Concomitant Medications
When CIALIS is coadministered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating treatment with CIALIS, and CIALIS should be initiated at the lowest recommended dose (see PRECAUTIONS).
For patients taking concomitant potent inhibitors of CYP3A4, such as ketoconazole or ritonavir, the maximum recommended dose of CIALIS is 10 mg, not to exceed once every 72 hours (see PRECAUTIONS).
Concomitant use of nitrates in any form is contraindicated (see CONTRAINDICATIONS).
Geriatrics - No dose adjustment is required in patients >65 years of age.
HOW SUPPLIED
CIALIS® (tadalafil) is supplied as follows:
Three strengths of film-coated, almond-shaped tablets are available in different sizes and different shades of yellow, and supplied in the following package sizes:
5-mg tablets debossed with "C 5"
Bottles of 30 NDC 0002-4462-30
10-mg tablets debossed with "C 10"
Bottles of 30 NDC 0002-4463-30
20-mg tablets debossed with "C 20"
Bottles of 30 NDC 0002-4464-30
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Keep out of reach of children. Literature revised July 8, 2005 Manufactured for Lilly ICOS LLC by
Eli Lilly and Company Indianapolis, IN 46285, USA
Copyright © 2003, 2005, Lilly ICOS LLC. All rights reserved.
APA Reference
Staff, H.
(2008, December 17). Cialis Full Prescribing Information, HealthyPlace. Retrieved
on 2026, January 26 from https://www.healthyplace.com/sex/treatment/cialis-full-prescribing-information