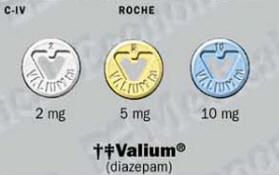
Valium (Diazepam) Patient Information
Find out why Valium is prescribed, side effects of Valium, Valium warnings, effects of Valium during pregnancy, more - in plain English.
Generic name: Diazepam
Brand name: Valium
Pronounced: VAL-ee-um
Why is Valium prescribed?
Valium is used in the treatment of anxiety disorders and for short-term relief of the symptoms of anxiety. It belongs to a class of drugs known as benzodiazepines.
It is also used to relieve the symptoms of acute alcohol withdrawal, to relax muscles, to relieve the uncontrolled muscle movements caused by cerebral palsy and paralysis of the lower body and limbs, to control involuntary movement of the hands (athetosis), to relax tight, aching muscles, and, along with other medications, to treat convulsive disorders such as epilepsy.
Most important fact about Valium
Valium can be habit-forming or addictive. You may experience withdrawal symptoms if you stop using this drug abruptly. Discontinue or change your dose only on your doctor's advice.
How should you take Valium?
Take this medication exactly as prescribed. If you are taking Valium for epilepsy, make sure you take it every day at the same time.
--If you miss a dose...
Take it as soon as you remember if it is within an hour or so of the scheduled time. If you do not remember until later, skip the dose you missed and go back to your regular schedule. Never take 2 doses at the same time.
--Storage instructions...
Store away from heat, light, and moisture.
What side effects may occur when taking Valium?
Side effects cannot be anticipated. If any develop or change in intensity, inform your doctor as soon as possible. Only your doctor can determine if it is safe for you to continue taking Valium.
-
More common side effects of Valium may include: Drowsiness, fatigue, light-headedness, loss of muscle coordination
-
Less common or rare side effects may include: Anxiety, blurred vision, changes in salivation, changes in sex drive, confusion, constipation, depression, difficulty urinating, dizziness, double vision, hallucinations, headache, inability to hold urine, low blood pressure, nausea, overstimulation, rage, seizures (mild changes in brain wave patterns), skin rash, sleep disturbances, slow heartbeat, slurred speech and other speech problems, stimulation, tremors, vertigo, yellowing of eyes and skin
-
Side effects due to rapid decrease in dose or abrupt withdrawal from Valium: Abdominal and muscle cramps, convulsions, sweating, tremors, vomiting
Why should this drug not be prescribed?
If you are sensitive to or have ever had an allergic reaction to Valium, you should not take this medication.
Do not take this medication if you have the eye condition known as acute narrow-angle glaucoma.
Anxiety or tension related to everyday stress usually does not require treatment with such a powerful drug as Valium. Discuss your symptoms thoroughly with your doctor.
Valium should not be prescribed if you are being treated for mental disorders more serious than anxiety.
Special warnings about Valium
Valium may cause you to become drowsy or less alert; therefore, you should not drive or operate dangerous machinery or participate in any hazardous activity that requires full mental alertness until you know how this drug affects you.
If you have liver or kidney problems, use this medication cautiously.
Possible food and drug interactions when taking Valium
Valium slows down the central nervous system and may intensify the effects of alcohol. Do not drink alcohol while taking this medication.
If Valium is taken with certain other drugs, the effects of either could be increased, decreased, or altered. It is especially important to check with your doctor before combining Valium with any of the following:
Antiseizure drugs such as Dilantin
Antidepressant drugs such as Elavil and Prozac
Barbiturates such as phenobarbital
Cimetidine (Tagamet)
Digoxin (Lanoxin)
Disulfiram (Antabuse)
Fluoxetine (Prozac)
Isoniazid (Rifamate)
Levodopa (Larodopa, Sinemet)
Major tranquilizers such as Mellaril and Thorazine
MAO inhibitors (antidepressant drugs such as Nardil)
Narcotics such as Percocet
Omeprazole (Prilosec)
Oral contraceptives
Propoxyphene (Darvon)
Ranitidine (Zantac)
Rifampin (Rifadin)
Special information if you are pregnant or breastfeeding
Do not take Valium if you are pregnant or planning to become pregnant. There is an increased risk of birth defects.
If this medication is essential to your health, your doctor may advise you to discontinue breastfeeding until your treatment is finished.
Recommended dosage for Valium
ADULTS
Treatment of Anxiety Disorders and Short-Term Relief of the Symptoms of Anxiety
The usual dose, depending upon severity of symptoms, is 2 milligrams to 10 milligrams 2 to 4 times daily.
Acute Alcohol Withdrawal
The usual dose is 10 milligrams 3 or 4 times during the first 24 hours, then 5 milligrams 3 or 4 times daily as needed.
Relief of Muscle Spasm
 The usual dose is 2 milligrams to 10 milligrams 3 or 4 times daily.
The usual dose is 2 milligrams to 10 milligrams 3 or 4 times daily.
Convulsive Disorders
The usual dose is 2 milligrams to 10 milligrams 2 to 4 times daily.
CHILDREN
Valium should not be given to children under 6 months of age. The usual starting dose for children over 6 months is 1 to 2.5 milligrams 3 or 4 times a day. Your doctor may increase the dosage gradually if needed.
OLDER ADULTS
The usual dosage is 2 milligrams to 2.5 milligrams once or twice a day, which your doctor will increase as needed. Your doctor will limit the dosage to the smallest effective amount because older people are more apt to become oversedated or uncoordinated.
Overdosage
Any medication taken in excess can have serious consequences. If you suspect a Valium overdose, seek medical attention immediately.
- Symptoms of Valium overdose may include: Coma, confusion, diminished reflexes, sleepiness
Detailed Info on Signs, Symptoms, Causes, Treatments of Anxiety Disorders
Detailed Info on Signs, Symptoms, Causes, Treatments of Addictions
APA Reference
Staff, H.
(2009, January 3). Valium (Diazepam) Patient Information, HealthyPlace. Retrieved
on 2024, May 5 from https://www.healthyplace.com/other-info/psychiatric-medications/valium-diazepam-patient-information