Halcion (Triazolam) Patient Information
Find out why Halcion is prescribed, side effects of Halcion, Halcion warnings, effects of Halcion during pregnancy, more - in plain English.
Generic name: Triazolam
Brand name: Halcion
Pronounced: HAL-see-on
Full Halcion (Triazolam) Prescription Information
Why is Halcion prescribed?
Halcion is used for short-term treatment of insomnia. It is a member of the benzodiazepine class of drugs, many of which are used as tranquilizers.
Most important fact about Halcion
Sleep problems are usually temporary, requiring treatment for only a short time, usually 1 or 2 days and no more than 1 to 2 weeks. Insomnia that lasts longer than this may be a sign of another medical problem. If you find you need this medicine for more than 7 to 10 days, be sure to check with your doctor.
How should you take Halcion?
Take this medication exactly as directed; never take more than your doctor has prescribed.
---If you miss a dose...
Take Halcion only as needed.
---Storage instructions...
Keep this medication in the container it came in, tightly closed, and out of reach of children. Store it at room temperature.
What side effects may occur using Halcion?
Side effects cannot be anticipated. If any develop or change in intensity, inform your doctor as soon as possible. Only your doctor can determine if it is safe for you to continue taking Halcion.
-
More common side effects of Halcion may include: Coordination problems, dizziness, drowsiness, headache, light-headedness, nausea/vomiting, nervousness
-
Less common or rare side effects may include: Aggressiveness, agitation, behavior problems, burning tongue, changes in sexual drive, chest pain, confusion, congestion, constipation, cramps/pain, delusions, depression, diarrhea, disorientation, dreaming abnormalities, drowsiness, dry mouth, exaggerated sense of well-being, excitement, fainting, falling, fatigue, hallucinations, impaired urination, inappropriate behavior, incontinence, inflammation of the tongue and mouth, irritability, itching, loss of appetite, loss of sense of reality, memory impairment, memory loss (e.g. traveler's amnesia), menstrual irregularities, morning "hangover" effects, muscle spasms in the shoulders or neck, nightmares, rapid heart rate, restlessness, ringing in the ears, skin inflammation, sleep disturbances including insomnia, sleepwalking, slurred or difficult speech, stiff awkward movements, taste changes, tingling or pins and needles, tiredness, visual disturbances, weakness, yellowing of the skin and whites of the eyes
Why should Halcion not be prescribed?
You should not take this drug if you are pregnant or if you have had an allergic reaction to it or to other benzodiazepine drugs such as Valium.
Also avoid Halcion if you are taking the antifungal medications Nizoral or Sporanox, or the antidepressant Serzone.
Special warnings about Halcion
When Halcion is used every night for more than a few weeks, it loses its effectiveness to help you sleep. This is known as tolerance. Also, it can cause dependence, especially when it is used regularly for longer than a few weeks or at high doses.
Abrupt discontinuation of Halcion should be avoided, since it has been associated with withdrawal symptoms (convulsions, cramps, tremor, vomiting, sweating, feeling ill, perceptual problems, and insomnia). A gradual dosage tapering schedule is usually recommended for patients taking more than the lowest dose of Halcion for longer than a few weeks. The usual treatment period is 7 to 10 days.
If you develop unusual and disturbing thoughts or behavior---including increased anxiety or depression---during treatment with Halcion, you should discuss them with your doctor immediately.
"Traveler's amnesia" has been reported by patients who took Halcion to induce sleep while traveling. To avoid this condition, do not take Halcion on an overnight airplane flight of less than 7 to 8 hours.
You may suffer increased anxiety during the daytime while taking Halcion.
When you first start taking Halcion, until you know whether the medication will have any "carry over" effect the next day, use extreme care while doing anything that requires complete alertness such as driving a car or operating machinery.
After discontinuing the drug, you may experience a "rebound insomnia" for the first 2 nights---that is, insomnia may be worse than before you took the sleeping pill.
You should be aware that anterograde amnesia (forgetting events after an injury) has been associated with benzodiazepine drugs such as Halcion.
You should be cautious about using this drug if you have liver or kidney problems, lung problems, or a tendency to temporarily stop breathing while you are asleep.
Possible food and drug interactions when taking Halcion
Avoid alcoholic beverages and grapefruit juice.
If Halcion is taken with certain other drugs, the effects of either could be increased, decreased, or altered. It is especially important to check with your doctor before combining Halcion with the following:
Amiodarone (Cordarone)
Antidepressant medications, including "tricyclic" drugs such as Elavil and such MAO inhibitors as Nardil and Parnate
Antihistamines such as Benadryl and Tavist
Barbiturates such as phenobarbital and Seconal
Cimetidine (Tagamet)
Clarithromycin (Biaxin)
Cyclosporine (Sandimmune Neoral)
Diltiazem (Cardizem)
Ergotamine (Cafergot)
Erythromycin (E.E.S., PCE, E-Mycin, others)
Fluvoxamine (Luvox)
Isoniazid (Nydrazid)
Itraconazole (Nizoral)
Ketoconazole (Sporanox)
Narcotic painkillers such as Demerol
Major tranquilizers such as Mellaril and Thorazine
Nefazodone (Serzone)
Nicardipine (Cardene)
Nifedipine (Adalat)
Other tranquilizers such as BuSpar, Valium, and Xanax
Oral contraceptives
Paroxetine (Paxil)
Ranitidine (Zantac)
Seizure medications such as Dilantin and Tegretol
Sertraline (Zoloft)
Verapamil (Calan)
Special information if you are pregnant or breastfeeding
Since benzodiazepines have been associated with damage to the developing baby, you should not take Halcion if you are pregnant, think you may be pregnant, or are planning to become pregnant; or if you are breastfeeding.
Recommended dosage for Halcion
ADULTS
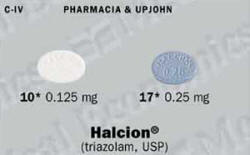 The usual dose is 0.25 milligram before bedtime. The dose should never be more than 0.5 milligram.
The usual dose is 0.25 milligram before bedtime. The dose should never be more than 0.5 milligram.
CHILDREN
Safety and effectiveness for children under the age of 18 have not been established.
OLDER ADULTS
To decrease the possibility of oversedation, dizziness, or impaired coordination, the usual starting dose is 0.125 milligram. This may be increased to 0.25 milligram if necessary.
Overdosage of Halcion
Any medication taken in excess can have serious consequences. Severe overdosage of Halcion can be fatal. If you suspect an overdose, seek medical help immediately.
- Symptoms of Halcion overdose may include: Apnea (temporary cessation of breathing), coma, confusion, excessive sleepiness, problems in coordination, seizures, shallow or difficult breathing, slurred speech
Full Halcion (Triazolam) Prescription Information
Extensive information on Anxiety Disorders, signs, symptoms, causes, treatment
APA Reference
Staff, H.
(2009, January 4). Halcion (Triazolam) Patient Information, HealthyPlace. Retrieved
on 2024, April 23 from https://www.healthyplace.com/other-info/psychiatric-medications/halcion-triazolam-patient-information